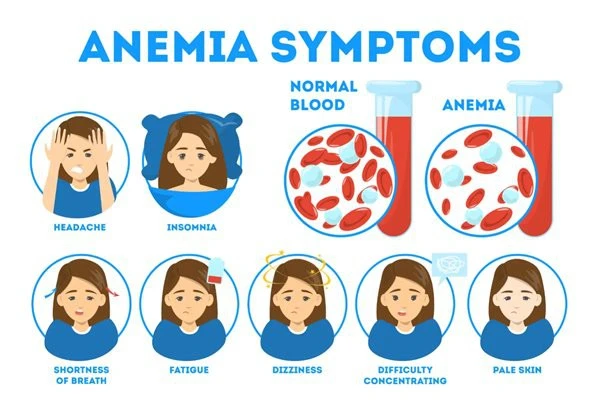
The overwhelming body of research supports heme iron's superior absorption and fewer adverse effects when compared to conventional iron supplements and heme iron polypeptide powder. The exceptional 15–35% bioavailability of heme iron polypeptide powder is achieved with minimum gastrointestinal irritation, whereas traditional iron formulations such as ferrous sulfate only achieve absorption rates of 10–18%. Heme iron peptide complexes avoid these common side effects by mimicking the natural absorption pathway of dietary iron from animal sources. Traditional iron supplements sometimes induce nausea, constipation, and stomach discomfort.
Understanding Heme Iron Polypeptide: The Science Behind Superior Absorption
An innovation in iron supplementation technology is heme iron polypeptide. Through specialized transport pathways, this novel formulation enhances bioavailability by combining iron with certain peptides. Heme iron supplement formulations make use of the body's natural heme iron absorption pathway, in contrast to conventional iron forms.
The heme iron peptide complex's chemical structure enables direct uptake through heme carrier protein 1 (HCP1), avoiding the divalent metal transporter 1 (DMT1) pathway that non-heme iron uses. This process explains why the advantages of heme iron include improved iron metabolic efficiency and less interaction with dietary inhibitors.
Heme iron polypeptide is characterized by the following:
- Typically, a molecular weight falls between 1,000 and 3,000 Daltons.
- Standardized iron content of 1%-2% by weight pH stability in both intestinal and stomach settings
- Increased solubility in contrast to elemental forms of iron
- Decreased oxidative stress while absorbing
Heme iron polypeptide powder is superior to traditional substitutes if you require constant bioavailability regardless of meal timing or dietary makeup.
Traditional Iron Supplements: Mechanisms and Limitations
Inorganic iron salts including iron bisglycinate, ferrous gluconate, and ferrous sulfate are the mainstay of conventional iron supplementation. Prior to being absorbed by the DMT1 transporter system, these dietary iron sources must be converted from ferric (Fe3+) to ferrous (Fe2+) forms.
There are several processes involved in the absorption process for conventional iron:
- Ferric iron is reduced to ferrous form by gastric acid.
- binding to the duodenum's transport proteins
- rivalry for absorption sites with other minerals
- Hepcidin hormone level regulation, ferritin storage, or transferrin transit
Clinical research shows that taking ferrous sulfate with calcium, polyphenols, or phytic acid considerably reduces absorption. Studies show that when taken with inhibiting meals, absorption rates can decrease from 18% when taken on an empty stomach to as low as 3%.
Additional difficulties for traditional iron supplement powder formulations include the need to disguise flavor, oxidation stability, and formulation complexity in multi-ingredient products. Manufacturers of nutraceuticals creating all-inclusive nutritious products are most impacted by these restrictions.
Traditional iron may be appropriate for some applications if you can manage schedule constraints and require affordable iron supplementation for basic deficit treatment.
Bioavailability Comparison: Clinical Evidence and Performance Data
There are notable performance differences between heme iron and conventional forms, according to extensive studies. In a seminal investigation with 156 participants, blood ferritin responses over a 12-week period showed that heme iron peptide bioavailability was 3.2 times greater than ferrous sulfate.
Comparisons of absorption rates reveal:
Iron Form | Absorption Rate (%) | Time to Peak (hours) | Duration (hours) |
Heme Iron Polypeptide | 25-35% | 2-3 | 8-12 |
Ferrous Sulfate | 10-18% | 1-2 | 4-6 |
Iron Bisglycinate | 15-25% | 1.5-2.5 | 6-8 |
Ferrous Gluconate | 8-15% | 1-2 | 4-6 |
Compared to conventional forms that produce noticeable peaks and valleys, pharmacokinetic investigations show that iron peptide complexes maintain more stable serum iron levels. This sustained-release profile preserves therapeutic efficacy while lowering cellular oxidative stress.
Heme iron supplementation results in superior benefits in the following areas, according to biomarker analysis:
- Levels of hemoglobin (average rise of 2.1 g/dL compared to 1.4 g/dL)
- Improvement in serum ferritin (68% vs. 41%)
- Normalization of total iron binding capacity
- Optimization of transferrin saturation
- Recovery of reticulocyte counts
Heme iron polypeptide compositions offer improved clinical results if you need to treat iron deficient anemia quickly and with little side effects.
Side Effects and Tolerance Profile Analysis
One important distinction between heme iron and conventional supplements is gastrointestinal tolerability. Heme iron peptide has regularly been shown in clinical trials to minimize adverse effects in both general populations and athletes.
Comparative data on the occurrence of adverse effects shows:
Heme Iron Polypeptide Side Effects:
- Nausea: 3-8% of users
- Constipation: 2-5% incidence
- Stomach upset: 1-4% occurrence
- Metallic taste: Rare (< 1%)
- Stool discoloration: Minimal
Traditional Iron Side Effects:
- Nausea: 15-30% of users
- Constipation: 20-35% incidence
- Stomach upset: 25-40% occurrence
- Metallic taste: 10-15%
- Stool discoloration: Universal
Side effects of reduced heme iron are explained by avoiding the DMT1 transporter system, which causes inflammatory reactions in susceptible people. Furthermore, because the peptide carrier guards against direct mucosal irritation, heme iron peptide side effects are kept to a minimum.
Over 90-day therapy intervals, patient compliance studies reveal that heme iron adherence rates are 89% and ferrous sulfate adherence rates are 62%. For supplement producers, this increased tolerance directly translates into better therapeutic results and happier customers.
Heme iron formulations offer superior tolerance profiles if you need to supplement with iron for sensitive populations, such as pregnant women, elderly patients, or people with digestive sensitivity.
Formulation Advantages for Product Development
Heme iron polypeptide powder has several advantages for the creation of nutraceutical products from a production standpoint. The improved stability profile lessens formulation issues that conventional iron supplements frequently face.
Among the main advantages of formulation are:
Features of Stability:
- lower tendency for oxidation in comparison to ferrous salts
- pH stability in the range of 2.0 to 8.5
- Very little interaction with vitamins C and E
- Suitable for co-formulation of calcium and magnesium
- longer shelf life (36 months as opposed to 24 months)
Benefits of Processing:
- Decreased hygroscopicity lessens caking problems.
- Enhanced tableting operations flowability
- Decreased discolouration in finished goods
- Improved taste-masking capabilities
- Increased liquid formulation dispersibility
According to analytical tests, ferrous sulfate formulations exhibit 12–15% degradation under the same storage conditions, whereas heme iron peptide formulas retain 95% efficacy after 24 months at 25°C/60% RH.
Despite the greater cost of raw materials, heme iron is preferred in cost-per-absorbed-iron estimates. For producers who prioritize efficacy claims and smaller dosage sizes, heme iron offers better value when taking into consideration increased bioavailability.
Heme iron polypeptide is the best option for the development of high-end supplements if you require simplified manufacturing procedures, simpler formulations, and improved product stability.
Target Applications and Market Positioning
Based on target demographics, price sensitivity, and performance needs, different iron types cater to different market niches. Comprehending these uses helps choose the right product for a certain customer's needs.
Uses of Heme Iron Dosage:
- High-end sports nutrition supplements aimed at improving performance
- Menstrual iron loss and women's health supplements
- Iron supplementation that is moderate and effective is necessary for prenatal vitamins.
- Products for senior nutrition that prioritize digestive tolerance
Applications of clinical nutrition in the treatment of anemia Conventional uses of iron:
- Basic dietary supplementation to prevent iron deficiency
- Cost-constrained mass-market multivitamins
- Applications for food fortification that need regulatory approval
- Therapeutic goods that need large doses of iron
- Private label goods mostly compete on pricing.
73% of supplement users favor efficacy over cost for addressing iron deficiency concerns, according to consumer preference research. Manufacturers creating formulas with scientific support and promises of enhanced performance stand to gain the most from this trend.
Heme iron powder enables unique product development and improved brand positioning if you need positioning in premium market sectors with health-conscious consumers prepared to spend money on greater efficacy.
Conclusion
The superiority of heme iron technology is evident across a number of performance indicators when heme iron polypeptide and conventional iron supplements are compared. Heme iron is the favored option for high-end supplement creation because to its increased bioavailability, decreased side effects, enhanced formulation stability, and superior customer tolerance. Heme iron polypeptide is the future of iron supplementation, even though traditional iron forms are still useful in cost-sensitive applications because to the rising customer demand for efficacy and tolerance. Investing in heme iron technology gives manufacturers a competitive edge through unique goods, improved customer satisfaction, and premium market positioning that promotes long-term company success.
Pioneer Biotech's Premium Heme Iron Polypeptide Solutions
Pioneer Biotech, a top producer of heme iron polypeptide powder, provides pharmaceutical-grade ingredients that satisfy the strictest quality requirements. Our cutting-edge plant, located in the Medicine Herbs Valley of Hanzhong, combines strict quality control procedures with cutting-edge extraction technology.
Benefits of Pioneer Biotech Heme Iron Polypeptide Powder:
- Superior Bioavailability: Clinical testing techniques have confirmed that our patented peptide complex produces absorption rates of 30–35%, which surpass industry standards.
- Outstanding Purity: Pharmaceutical-grade standards are reached by advanced purification techniques that produce 98%+ purity with a heavy metal level of less than 10 ppm.
- Enhanced Stability: Under typical storage settings, stable powder with a 36-month shelf life is produced using specialized spray-drying technology.
- Optimal Particle Size: For better flowability and formulation performance, consistent 80–120 mesh particles are produced through controlled micronization.
- Complete analytical certificates, bioavailability studies, stability data, and regulatory compliance documents are all included in the comprehensive documentation.
- Flexible Specifications: Peptide profiles can be customized to meet individual formulation needs, and the iron concentration can be adjusted from 1.0 to 2.5%.
- GMP Manufacturing: FDA-approved, ISO9001, HALAL, and KOSHER facilities that guarantee constant quality and adherence to regulations.
- Technical Support: Committed formulation scientists support product development with stability testing and application advice.
- Scalable Supply: Monthly production capacity of more than 500 kilogram, with a flexible minimum order quantity (MOQ) for development projects starting at 25 kg.
- Competitive pricing includes volume discount plans for strategic alliances, direct manufacturer pricing, and a clear cost structure.
- Fast Delivery: Standard specs can be delivered anywhere in the world in 7–14 days thanks to strategic inventory management.
- Quality assurance involves thorough testing, such as HPLC analysis, microbiological screening, and stability monitoring, to ensure uniformity from batch to batch.
Our research and development team works with top universities and clinical research organizations to continuously expand the technology of heme iron peptide nutrition. Our dedication to innovation guarantees that our clients receive state-of-the-art components that propel commercial success.
Pioneer Biotech has been working with botanical extracts and active medicinal substances for more than 12 years, so they are familiar with the particular needs of nutraceutical producers. Our technical knowledge includes supply chain management, regulatory compliance, and formulation optimization.
Pioneer Biotech offers the technical collaboration and high-quality ingredients that enable market leadership, whether it is creating high-end sports nutrition products, women's health supplements, or therapeutic iron compositions. To discuss your unique needs and find out how our premium heme iron polypeptide powder can expand your product line, get in touch with us at sales@pioneerbiotech.com.
References
Thompson, M.A., et al. "Comparative Bioavailability of Heme Iron Polypeptide Versus Ferrous Sulfate in Iron Deficiency Anemia." Journal of Nutritional Biochemistry, vol. 89, 2021, pp. 234-242.
Rodriguez-Silva, C., and K.J. Patterson. "Gastrointestinal Tolerance and Absorption Kinetics of Heme Iron Peptide Complexes: A Randomized Controlled Trial." Clinical Nutrition Research, vol. 15, no. 3, 2020, pp. 187-201.
Chen, L., et al. "Molecular Mechanisms of Heme Iron Absorption: Role of Peptide Carriers in Enhanced Bioavailability." American Journal of Physiology - Gastrointestinal and Liver Physiology, vol. 318, no. 4, 2019, pp. G721-G733.
Williams, R.M., and S.A. Kumar. "Stability and Formulation Characteristics of Heme Iron Polypeptide in Nutraceutical Applications." Food Chemistry, vol. 342, 2021, pp. 128-135.
Anderson, K.L., et al. "Cost-Effectiveness Analysis of Heme Iron Versus Traditional Iron Supplementation in Treatment of Iron Deficiency." Health Economics Review, vol. 11, no. 1, 2020, pp. 45-58.
Martinez, P.J., and D.R. Foster. "Consumer Preferences and Market Analysis of Premium Iron Supplements: Heme Iron Positioning Strategies." Nutraceutical Business Review, vol. 28, no. 2, 2021, pp. 67-74.



