How Can Pure Lactoferrin Powder Enhance Your Product's Market Appeal?
In today's competitive marketplace, manufacturers across pharmaceutical, nutraceutical, and functional food industries are constantly seeking innovative ingredients that can differentiate their products and capture consumer attention. Pure lactoferrin powder emerges as a game-changing bioactive ingredient that not only enhances nutritional profiles but also significantly boosts market appeal through its scientifically-proven health benefits and premium positioning. This naturally occurring glycoprotein, when properly processed and standardized, offers manufacturers a unique opportunity to create products that resonate with health-conscious consumers while commanding premium pricing in increasingly crowded markets.
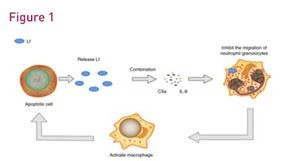
Unlocking Premium Market Positioning Through Bioactive Innovation
Scientific Foundation and Bioactivity Preservation
Pure lactoferrin powder represents a sophisticated approach to functional ingredient manufacturing, where advanced freeze-drying technology preserves the natural structure and bioactivity of this remarkable protein. The freeze dried lactoferrin production process ensures that the delicate iron-binding properties and antimicrobial characteristics remain intact, making it an ideal choice for manufacturers seeking scientifically-backed ingredients. This preservation method is crucial because lactoferrin's bioactivity directly correlates with its market value and consumer appeal. Unlike conventional protein powders, pure lactoferrin powder maintains its native conformation, ensuring that the multifunctional properties that make it so valuable remain active throughout product shelf life. The scientific rigor behind freeze-drying technology demonstrates to consumers and industry professionals that the ingredient has been processed with the highest standards, supporting premium positioning strategies. Manufacturers who incorporate pure lactoferrin powder into their formulations can confidently communicate the scientific sophistication of their products, appealing to educated consumers who understand the importance of bioactive preservation in nutritional supplements.
Regulatory Compliance and Quality Assurance
The market appeal of products containing pure lactoferrin powder is significantly enhanced by the stringent regulatory compliance and quality assurance measures associated with professional manufacturing. FDA registered facilities ensure that freeze dried lactoferrin meets the highest safety and quality standards, providing manufacturers with the confidence needed to enter regulated markets. Certifications including HACCP, ISO22001, EU USDA Organic, Kosher, and Halal credentials create multiple market entry opportunities across diverse consumer segments and geographical regions. These certifications are not merely regulatory requirements but powerful marketing tools that communicate quality, safety, and inclusivity to potential customers. The gluten-free, non-GMO, and allergen-free profile of pure lactoferrin powder further expands its market appeal, addressing the growing consumer demand for clean-label products. Manufacturers can leverage these quality credentials to justify premium pricing while building trust with distributors and end consumers. The comprehensive certification portfolio demonstrates a commitment to excellence that resonates particularly well in international markets where regulatory compliance is paramount for market access and consumer acceptance.
Competitive Differentiation Through Purity Standards
Market differentiation becomes increasingly important as more companies enter the functional ingredient space, and pure lactoferrin powder offers exceptional opportunities for competitive advantage through its superior purity standards. The absence of parabens, artificial colors, and BSE/TSE contamination creates a clean product profile that appeals to quality-conscious manufacturers and their customers. This purity extends beyond basic safety requirements to encompass the preservation of functional properties that directly impact product efficacy. When manufacturers choose freeze dried lactoferrin with verified purity credentials, they can confidently position their products as premium offerings in markets where quality commands higher prices. The CAS number 146897-68-9 provides scientific traceability that supports regulatory submissions and quality documentation, essential for pharmaceutical and nutraceutical applications. Manufacturers utilizing pure lactoferrin powder can develop marketing strategies that emphasize both the scientific rigor of their ingredient selection and the superior outcomes that result from using highest-quality raw materials. This positioning strategy is particularly effective in markets where consumers are willing to pay premium prices for products that deliver proven benefits through scientifically-validated ingredients.
Expanding Market Reach Through Versatile Applications
Pharmaceutical and Clinical Applications
The pharmaceutical industry represents one of the most lucrative markets for pure lactoferrin powder applications, where its bioactive properties translate directly into therapeutic benefits and market opportunities. Clinical applications of freeze dried lactoferrin include wound healing formulations, anti-inflammatory preparations, and antimicrobial treatments, each representing distinct market segments with specific regulatory requirements and pricing structures. Pharmaceutical manufacturers benefit from lactoferrin's well-documented safety profile and extensive research foundation, which supports regulatory submissions and clinical trial designs. The iron-binding properties of pure lactoferrin powder make it particularly valuable in specialized pharmaceutical applications where iron metabolism support is clinically indicated. Market appeal in pharmaceutical applications is enhanced by the ingredient's natural origin and biocompatibility, addressing growing preferences for biologics and naturally-derived therapeutics. Manufacturers developing pharmaceutical formulations with pure lactoferrin powder can target multiple therapeutic areas, from immune support to gastrointestinal health, creating diverse revenue streams and market opportunities. The suggested dosage range of 250mg to 500mg per day provides pharmaceutical formulators with flexibility in developing products that meet specific clinical objectives while maintaining cost-effectiveness and patient compliance.
Functional Food and Beverage Innovation
The functional food and beverage market presents exceptional growth opportunities for manufacturers incorporating pure lactoferrin powder into innovative product formulations that appeal to health-conscious consumers. Energy drinks enhanced with freeze dried lactoferrin offer superior nutritional profiles compared to conventional formulations, enabling manufacturers to command premium pricing while targeting fitness and wellness markets. Sports nutrition products benefit significantly from lactoferrin's iron metabolism support and antioxidant properties, creating products that appeal to athletes and active individuals seeking performance enhancement through superior nutrition. Meal replacement shakes incorporating pure lactoferrin powder provide complete protein profiles with added bioactive benefits, differentiating these products in increasingly competitive markets. The versatility of pure lactoferrin powder allows manufacturers to develop innovative beverage formulations that combine taste, nutrition, and functional benefits in ways that resonate with diverse consumer segments. Market research consistently demonstrates that consumers are willing to pay premium prices for functional beverages that deliver scientifically-proven health benefits, making pure lactoferrin powder an ideal ingredient for manufacturers seeking to optimize both product performance and profitability. The clean label profile of freeze dried lactoferrin supports marketing strategies that emphasize natural ingredients and minimal processing, addressing key consumer preferences in functional food markets.
Infant Nutrition and Specialized Markets
Infant formula represents one of the most specialized and high-value markets for pure lactoferrin powder applications, where safety, efficacy, and regulatory compliance create substantial barriers to entry while rewarding successful manufacturers with premium pricing opportunities. Lactoferrin's natural presence in human breast milk makes it an essential component in infant nutrition formulations, providing manufacturers with scientifically-validated justification for inclusion in premium infant formula products. The freeze dried lactoferrin production process ensures that the bioactive properties crucial for infant health remain intact, supporting healthy immune system development and iron absorption in growing infants. Manufacturers developing infant nutrition products with pure lactoferrin powder can position their formulations as closely mimicking the nutritional profile of human breast milk, a powerful marketing message that resonates with health-conscious parents. Regulatory requirements for infant formula are among the most stringent in the food industry, making the comprehensive certification portfolio of quality pure lactoferrin powder particularly valuable for manufacturers seeking to enter or expand in these markets. The specialized nature of infant nutrition creates opportunities for long-term customer relationships and brand loyalty, as parents who trust a particular formula brand often continue using related products as their children grow. Market appeal in infant nutrition is enhanced by the natural origin and safety profile of pure lactoferrin powder, addressing parental concerns about artificial additives and synthetic ingredients in infant food products.
Maximizing Commercial Success Through Strategic Implementation
Supply Chain Optimization and Market Responsiveness
Successful implementation of pure lactoferrin powder in commercial products requires sophisticated supply chain management that ensures consistent quality, reliable availability, and cost-effective procurement strategies. Manufacturers benefit from working with suppliers who maintain ready stock in local warehouses, enabling rapid response to market demands and minimizing inventory carrying costs while ensuring product availability during peak demand periods. The freeze dried lactoferrin supply chain involves complex coordination between raw material sourcing, processing facilities, quality control laboratories, and distribution networks, making supplier selection a critical factor in commercial success. Prompt and secure shipment capabilities become essential when manufacturers need to respond quickly to market opportunities or customer orders, particularly in international markets where logistics complexity can impact competitiveness. Strategic partnerships with pure lactoferrin powder suppliers who offer comprehensive paperwork support facilitate smooth customs clearance and regulatory compliance in global markets. Manufacturers who establish reliable supply relationships can confidently develop marketing strategies and customer commitments knowing that their ingredient supply will support business growth. The availability of gift samples enables manufacturers to conduct thorough testing and validation before committing to large-scale procurement, reducing risk while ensuring that the pure lactoferrin powder meets specific formulation requirements and quality standards.
OEM Services and Customization Opportunities
The availability of comprehensive OEM services significantly enhances the market appeal of pure lactoferrin powder by enabling manufacturers to develop customized solutions that meet specific market requirements and brand positioning strategies. Customized packaging options allow manufacturers to create unique product presentations that differentiate their offerings in competitive markets while maintaining the quality and bioactivity of freeze dried lactoferrin. Private labeling services enable manufacturers to develop products under their own brand names while leveraging the expertise and capabilities of specialized ingredient suppliers. Formulation customization provides opportunities to develop unique product profiles that combine pure lactoferrin powder with complementary ingredients, creating synergistic effects that enhance both efficacy and market appeal. Manufacturers utilizing OEM services can accelerate time-to-market while reducing development costs and technical risks associated with ingredient handling and processing. The flexibility offered through OEM partnerships enables manufacturers to respond quickly to changing market demands and customer preferences without significant capital investment in specialized processing equipment. Strategic use of OEM services allows manufacturers to focus on their core competencies in marketing, distribution, and customer relationships while ensuring that their products meet the highest technical and quality standards. This approach is particularly valuable for manufacturers entering new markets or developing innovative product categories where technical expertise and regulatory knowledge are critical for success.
Quality Assurance and Customer Confidence
Building customer confidence through comprehensive quality assurance programs becomes essential when manufacturers incorporate pure lactoferrin powder into products that command premium pricing and target quality-conscious consumers. The multi-layered certification portfolio including ISO9001, ISO22000, HACCP, and organic certifications provides manufacturers with powerful tools for communicating quality and safety to both business customers and end consumers. SGS verification adds independent third-party validation that supports marketing claims and regulatory submissions while building trust with distributors and customers who require documented proof of quality standards. Freeze dried lactoferrin manufacturing processes that meet FDA registration requirements provide additional assurance for manufacturers targeting North American markets where regulatory compliance is paramount. The availability of comprehensive testing documentation and certificates of analysis enables manufacturers to support their own quality management systems while providing customers with detailed information about product specifications and quality attributes. Cooperation with internationally recognized testing organizations ensures that pure lactoferrin powder meets global quality standards, facilitating market entry and customer acceptance in diverse geographical markets. Manufacturers who prioritize quality assurance can build long-term customer relationships based on trust and reliability, creating sustainable competitive advantages that support premium pricing and market growth. The investment in quality systems and documentation pays dividends through reduced customer complaints, regulatory compliance, and enhanced brand reputation in markets where quality is a primary purchasing criterion.
Conclusion
Pure lactoferrin powder represents a transformative opportunity for manufacturers seeking to enhance their product's market appeal through scientifically-validated bioactive ingredients. The combination of advanced freeze-drying technology, comprehensive quality certifications, and versatile application possibilities creates unique positioning opportunities that resonate with health-conscious consumers and quality-focused business customers. From pharmaceutical applications to infant nutrition and functional foods, pure lactoferrin powder enables manufacturers to develop premium products that command higher margins while delivering proven health benefits that build customer loyalty and market differentiation.
As a leading pure lactoferrin powder factory with strategic location in Hanzhong's "Herb Valley" of Qinling Mountains, Shaanxi Pioneer Biotech Co., Ltd. combines unique resource advantages with world-class manufacturing capabilities to serve global markets. Our pure lactoferrin powder supplier credentials include nine authoritative international certifications and modern production facilities capable of delivering over 3,000 tons annually. As your trusted pure lactoferrin powder manufacturer, we offer comprehensive OEM services, prompt global logistics, and complete quality documentation to support your success. Whether you're seeking pure lactoferrin powder wholesale opportunities or customized formulation solutions, our expertise in standardized herbal extracts and natural active ingredients ensures your products achieve maximum market appeal. Connect with our team today at sales@pioneerbiotech.com to discover how our pure lactoferrin powder can transform your product portfolio and accelerate your market growth in the competitive landscape of functional ingredients.
References
1. García-Montoya, I.A., Cendón, T.S., Arévalo-Gallegos, S., & Rascón-Cruz, Q. (2012). Lactoferrin a multiple bioactive protein: An overview. Biochimica et Biophysica Acta - General Subjects, 1820(3), 226-236.
2. Legrand, D., Pierce, A., Elass, E., Carpentier, M., Mariller, C., & Mazurier, J. (2008). Lactoferrin structure and functions. Advances in Experimental Medicine and Biology, 606, 163-194.
3. Brock, J.H. (2002). The physiology of lactoferrin. Biochemistry and Cell Biology, 80(1), 1-6.
4. Tomita, M., Wakabayashi, H., Shin, K., Yamauchi, K., Yaeshima, T., & Iwatsuki, K. (2009). Twenty-five years of research on bovine lactoferrin applications. Biochimie, 91(1), 52-57.



