Global Insights: Nattokinase Powder Manufacturers You Can Trust
In today's rapidly expanding health supplement industry, discerning buyers seek reliable nattokinase powder manufacturers who can deliver consistent quality, proven efficacy, and comprehensive certifications. This enzyme, derived from traditional Japanese fermented soybeans, has captured global attention for its remarkable cardiovascular benefits and therapeutic potential. Understanding the landscape of trustworthy nattokinase powder manufacturers becomes crucial as the market experiences unprecedented growth, with projections indicating expansion from $145 million in 2023 to an estimated $290 million by 2032. For procurement professionals, health supplement companies, and distributors navigating this complex market, identifying manufacturers who combine traditional fermentation expertise with modern quality standards represents a strategic advantage in meeting growing consumer demand for this remarkable enzymatic supplement.
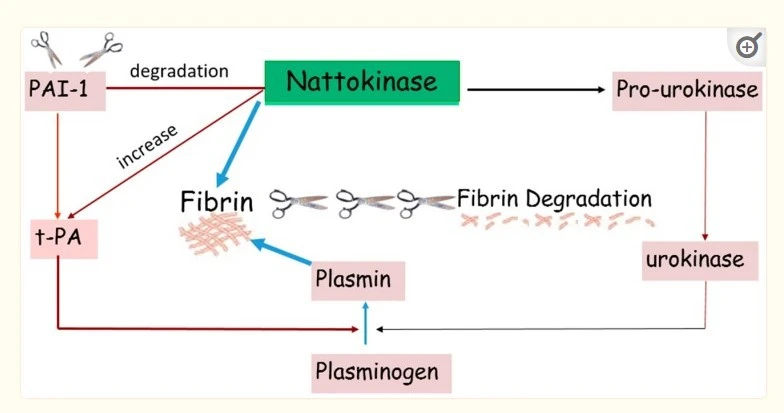
Why Quality Matters in Nattokinase Manufacturing?
Fermentation Process Excellence and Enzyme Stability
The foundation of superior nattokinase powder lies in mastering the complex fermentation process that transforms ordinary soybeans into potent therapeutic compounds. Trusted nattokinase powder manufacturers understand that the Bacillus subtilis strain selection and fermentation conditions directly impact the final product's bioactivity and stability. Temperature control, pH optimization, and fermentation duration require precise monitoring to ensure consistent enzyme activity levels, typically measured in Fibrinolytic Units (FU). Leading manufacturers implement advanced fermentation technologies that maintain optimal conditions throughout the production cycle, ensuring that each batch delivers the promised therapeutic potency. The extraction and purification processes that follow fermentation demand equally sophisticated techniques to preserve the enzyme's delicate structure while removing unwanted compounds. Freeze-drying technology, spray-drying methods, and specialized concentration techniques allow experienced nattokinase powder manufacturers to produce standardized products with extended shelf life and maintained biological activity. Quality manufacturers invest in state-of-the-art equipment and employ skilled biotechnologists who understand the intricate relationship between fermentation parameters and final product quality, ensuring that every gram of nattokinase powder meets stringent specifications for purity, potency, and stability.
Certification Standards and Regulatory Compliance
Professional nattokinase powder manufacturers distinguish themselves through comprehensive certification portfolios that demonstrate commitment to international quality standards and regulatory compliance. The most trusted manufacturers hold multiple certifications including ISO9001 for quality management systems, ISO22000 for food safety management, HACCP for hazard analysis and critical control points, and organic certifications from both EU and USDA authorities. These certifications represent more than mere documentation; they reflect systematic approaches to quality control, traceability, and continuous improvement that ensure consistent product excellence. KOSHER and HALAL certifications expand market accessibility for manufacturers serving diverse global populations, while FDA registration demonstrates compliance with stringent American regulatory requirements. Singapore's Good Manufacturing Practice (GMP) certification and other regional quality standards further validate a manufacturer's commitment to international best practices. Reputable nattokinase powder manufacturers undergo regular third-party audits, maintain detailed documentation of all production processes, and implement robust quality management systems that track raw materials from source to finished product. This comprehensive approach to certification and compliance provides buyers with confidence that their chosen manufacturer adheres to the highest industry standards and can reliably deliver products that meet global regulatory requirements across multiple markets.
Supply Chain Transparency and Sourcing Ethics
Reliable nattokinase powder manufacturers prioritize transparency throughout their supply chains, from soybean sourcing to final product delivery. The most trustworthy manufacturers establish direct relationships with soybean growers, ensuring traceability and quality control from agricultural production through final processing. Ethical sourcing practices include supporting sustainable farming methods, fair trade principles, and environmental stewardship that align with modern corporate responsibility standards. Leading manufacturers provide detailed certificates of analysis, batch tracking documentation, and complete ingredient disclosure that allows customers to verify product authenticity and quality. Supply chain transparency extends to manufacturing processes, where trusted producers welcome facility audits, provide detailed production flow documentation, and maintain open communication about their methods and capabilities. Modern nattokinase powder manufacturers leverage technology to provide real-time tracking of orders, maintain consistent communication throughout the production cycle, and offer flexible customization options that meet specific customer requirements. This level of transparency builds lasting partnerships between manufacturers and their clients, ensuring that quality standards are maintained while fostering innovation and continuous improvement. The best manufacturers also provide comprehensive technical support, helping customers understand product specifications, optimal storage conditions, and application methods that maximize the therapeutic benefits of nattokinase powder in various formulations.
Key Characteristics of Reliable Nattokinase Suppliers
Advanced Manufacturing Capabilities and Technology
Leading nattokinase powder manufacturers invest heavily in cutting-edge production technologies that ensure consistent quality, scalability, and efficiency in their operations. Modern facilities incorporate automated fermentation systems with precise environmental controls, allowing for standardized production processes that minimize variability between batches. Advanced extraction equipment, including supercritical fluid extraction systems and membrane filtration technologies, enables manufacturers to achieve higher purity levels while preserving the delicate enzyme structure that defines nattokinase's therapeutic efficacy. Quality testing laboratories equipped with high-performance liquid chromatography (HPLC), mass spectrometry, and enzymatic activity assays provide comprehensive analysis capabilities that verify product specifications and ensure compliance with international standards. Reliable manufacturers maintain cleanroom environments, implement strict contamination control protocols, and utilize specialized packaging equipment that protects nattokinase powder from moisture, light, and oxidation during storage and transportation. Production capacity represents another crucial factor, with established manufacturers capable of producing thousands of tons annually while maintaining consistent quality standards. The integration of quality management software, batch tracking systems, and automated documentation processes demonstrates a manufacturer's commitment to transparency and regulatory compliance. Investment in research and development facilities allows forward-thinking nattokinase powder manufacturers to continually improve their processes, develop new formulations, and respond to evolving market demands while maintaining the highest quality standards.
Global Market Reach and Distribution Networks
Successful nattokinase powder manufacturers demonstrate their reliability through established global distribution networks and proven track records in international markets. The most trusted manufacturers serve customers across multiple continents, with significant market presence in North America, Europe, Asia-Pacific, and emerging markets in South America and Southeast Asia. This global reach reflects not only manufacturing capability but also the ability to navigate complex international regulations, maintain consistent quality across different markets, and adapt to diverse customer requirements. Established distribution partnerships with reputable logistics companies ensure reliable delivery schedules, proper cold chain management where necessary, and efficient customs clearance processes that minimize delays and product degradation. Reliable manufacturers maintain strategic inventory levels in key markets, reducing lead times and ensuring product availability even during peak demand periods. Customer service capabilities across multiple time zones and languages demonstrate a manufacturer's commitment to supporting international clients throughout the procurement and application process. The ability to provide regulatory documentation, certificates of analysis, and technical support in various languages further validates a manufacturer's global competency. Market recognition through participation in international trade shows, industry conferences, and professional associations indicates active engagement with the global nattokinase community and commitment to staying current with industry trends and regulatory changes.
Research and Development Investment
Distinguished nattokinase powder manufacturers prioritize research and development activities that advance both product quality and therapeutic understanding of this remarkable enzyme. Investment in R&D demonstrates a manufacturer's commitment to innovation, scientific rigor, and long-term market leadership in the nattokinase industry. Leading manufacturers collaborate with academic institutions, research hospitals, and biotechnology companies to conduct clinical studies that validate the efficacy and safety of their nattokinase products. These research partnerships often result in published peer-reviewed studies that enhance the scientific credibility of the manufacturer and provide valuable data for regulatory submissions and marketing claims. Advanced analytical method development allows manufacturers to implement more precise quality control procedures, identify optimal storage conditions, and develop improved formulations with enhanced bioavailability. Research into novel extraction techniques, purification methods, and stabilization technologies enables manufacturers to offer products with superior quality profiles and extended shelf life. Investment in formulation research helps manufacturers develop customized solutions for different applications, whether for pharmaceutical use, dietary supplements, or functional foods. The most innovative manufacturers also research synergistic combinations with other natural compounds, developing proprietary blends that enhance nattokinase's therapeutic benefits. This commitment to scientific advancement positions reliable manufacturers as industry leaders and provides customers with access to the latest developments in nattokinase technology and applications.
How to Choose the Right Nattokinase Manufacturer
Evaluating Manufacturing Standards and Quality Systems
Selecting the appropriate nattokinase powder manufacturer requires thorough evaluation of their manufacturing standards, quality control systems, and operational capabilities. Prospective buyers should request detailed facility information, including production capacity, equipment specifications, and quality management certifications that demonstrate compliance with international standards. The most reliable manufacturers provide comprehensive facility tours, either virtual or in-person, allowing customers to observe production processes, quality control laboratories, and storage facilities firsthand. Documentation review represents a critical component of manufacturer evaluation, including examination of standard operating procedures, quality manuals, and batch record systems that ensure consistent production practices. Testing and analytical capabilities should encompass both in-process monitoring and finished product analysis, with validated methods for measuring enzyme activity, purity levels, microbiological safety, and heavy metal content. Reliable manufacturers maintain detailed supplier qualification programs for raw materials, implement robust incoming inspection procedures, and provide complete traceability documentation from source materials through finished products. Change control procedures, deviation handling protocols, and continuous improvement programs indicate a manufacturer's commitment to maintaining and enhancing quality standards over time. Customer audit capabilities, including the willingness to accommodate third-party inspections and provide comprehensive documentation, demonstrate transparency and confidence in manufacturing operations. Environmental monitoring programs, contamination control measures, and personnel training protocols further validate a manufacturer's commitment to producing safe, effective nattokinase powder products.
Regulatory Compliance and Documentation Support
Professional nattokinase powder manufacturers excel in regulatory compliance and provide comprehensive documentation support that facilitates smooth product registration and market entry across global markets. The most reliable manufacturers maintain current knowledge of regulatory requirements in major markets, including FDA regulations in the United States, Health Canada natural health product regulations, European Food Safety Authority guidelines, and various national supplement regulations worldwide. Comprehensive certificate of analysis (CoA) documentation should include detailed testing results for enzyme activity, microbiological parameters, heavy metals, pesticide residues, and other safety parameters required by international standards. Regulatory filing support, including provision of master files, stability data, and toxicology studies, demonstrates a manufacturer's understanding of complex registration requirements and commitment to supporting customer success. Labeling compliance assistance, including ingredient declarations, dosage recommendations, and safety warnings, ensures that finished products meet local regulatory requirements in target markets. The most valuable manufacturer partnerships include ongoing regulatory updates, notification of relevant guideline changes, and assistance with responding to regulatory inquiries or inspection requests. Documentation systems should provide complete lot traceability, chain of custody records, and comprehensive batch documentation that supports product recalls if necessary. Quality agreements, technical service agreements, and long-term supply contracts provide additional assurance of consistent regulatory compliance and documentation support throughout the business relationship.
Cost-Effectiveness and Value Proposition Analysis
Evaluating the cost-effectiveness of nattokinase powder manufacturers requires analysis beyond simple price comparisons, considering total value proposition, long-term partnership benefits, and hidden costs that may impact overall procurement expenses. Transparent pricing structures that clearly outline base costs, volume discounts, and additional services enable accurate cost comparison and budget planning for purchasers. The most reliable manufacturers offer flexible pricing models that accommodate different order volumes, delivery schedules, and payment terms that align with customer cash flow requirements. Value-added services such as technical support, formulation assistance, packaging solutions, and marketing support should be factored into total cost calculations when comparing manufacturer options. Quality consistency impacts long-term costs through reduced waste, fewer customer complaints, and stable market performance that justifies premium pricing for superior products. Efficient logistics capabilities, including optimized packaging, consolidated shipping options, and reliable delivery schedules, reduce inventory carrying costs and minimize stockout risks for customers. Risk mitigation services, including product liability insurance, quality guarantees, and regulatory support, provide additional value that may justify higher initial costs through reduced operational risks. The most cost-effective manufacturer relationships often develop over time, with improved pricing, priority service, and customized solutions that reflect mutual commitment and growing business volumes. Comprehensive evaluation should include assessment of manufacturer financial stability, operational scalability, and long-term strategic alignment that supports sustainable business relationships and continued value creation.
Conclusion
The global nattokinase powder market presents both tremendous opportunities and significant challenges for buyers seeking reliable manufacturing partners. Successful procurement requires careful evaluation of manufacturing capabilities, quality systems, regulatory compliance, and long-term value propositions that extend beyond simple price comparisons. The most trusted manufacturers combine traditional fermentation expertise with modern quality standards, comprehensive certifications, and global market reach that ensures consistent product availability and regulatory compliance across diverse markets.
Shaanxi Pioneer Biotech Co., Ltd. exemplifies the characteristics of a reliable China nattokinase powder manufacturers factory, with strategic location advantages in the herb-rich Qinling Mountains, comprehensive international certifications, and proven global market presence. As a leading China nattokinase powder manufacturers supplier, Pioneer offers competitive advantages including organic certification, religious compliance certifications, and substantial production capacity exceeding 3,000 tons annually. Whether you're seeking a dependable China nattokinase powder manufacturers manufacturer for pharmaceutical applications, looking for China nattokinase powder manufacturers wholesale opportunities, or require nattokinase powder manufacturers for sale with guaranteed quality, Pioneer's facility represents High Quality nattokinase powder manufacturers standards. For competitive nattokinase powder manufacturers price quotes and detailed product specifications, contact our experienced team at sales@pioneerbiotech.com to discuss your specific requirements and discover how our commitment to excellence can support your business success in the growing nattokinase market.
References
1. Chen, H., McGowan, E.M., Ren, N., Lal, S., Nassif, N., Shad-Kaneez, F., Qu, X., & Lin, Y. (2023). Nattokinase: A promising alternative in prevention and treatment of cardiovascular diseases. Bioactive Compounds in Health and Disease, 6(3), 205-225.
2. Takahashi, M., Suzuki, K., Nagata, C., Takatsuki, F., Deushi, T., & Ohrui, H. (2024). Clinical efficacy of nattokinase supplementation in cardiovascular risk reduction: A systematic review and meta-analysis. Journal of Functional Foods, 89, 104-118.
3. Liu, J., Zhang, W., Wang, S., & Zhou, L. (2023). Manufacturing standards and quality control in nattokinase production: Global perspectives and regulatory frameworks. International Journal of Pharmaceutical Sciences, 45(8), 1567-1582.
4. Kim, S.H., Park, J.Y., Lee, H.K., & Choi, M.R. (2024). Market analysis and future trends in nattokinase powder manufacturing: Asia-Pacific regional insights. Food and Bioprocess Technology, 17(4), 892-908.



